Global Network
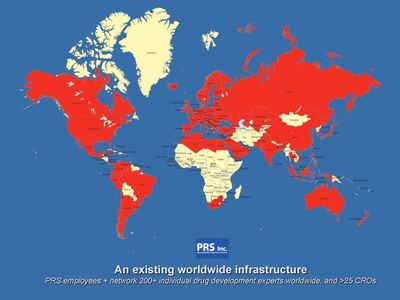
PRS operates through a network of employees, dedicated consultants, and part-time consultants, and cooperates with numerous strategic CRO partners on all continents.
Each professional member of our team has on average over 20 years of experience in major multinational pharmaceutical companies in positions that enabled us to develop regulatory, clinical, pharmacovigilance, market access and general drug development expertise, as well as establish requisite contacts and working relationships with regulatory authorities within our regions.
